

Site Search
Search within product
第768号 2025(R7).02-03発行
Click here for PDF version
§秋田県のタマネギ秋まき作型における肥効調節型肥料を用いた省力施肥技術
秋田県農業試験場 野菜・花き部
菅原 茂幸
§岩手県におけるブルーベリーの土壌管理と施肥法
National Federation of Agricultural Cooperative Associations, Iwate Headquarters
Horticulture Department, Horticulture Specialty Products Division
佐々木 仁
§土のはなし-第39回
慣行農業の養分源・化学肥料の課題
-原料の資源枯渇や生産のエネルギー問題-
前 ジェイカムアグリ株式会社
北海道支店 技術顧問
松中 照夫
Onion in Autumn Sowing in Akita Prefecture
肥効調節型肥料を用いた省力施肥技術
秋田県農業試験場 野菜・花き部
菅原 茂幸
Introduction
The area planted with onions in Akita Prefecture expanded to 83 ha in 2022 (Ministry of Agriculture, Forestry and Fisheries, 2023). There are two types of onion cultivation: spring sown in Hokkaido and fall sown west of Honshu. In our prefecture, which is located in the northeastern part of Japan, both types of onions are grown, but they have not yet developed into a major production area. The reasons for this are that the spring-sown type has a short period from planting to harvest, which tends to produce small balls, and that the growing and harvesting periods coincide with the rainy season, which tends to produce rotten balls due to disease. On the other hand, in the case of fall sowing, early planting results in excessive growth, which causes many cases of moss formation, while late planting results in small balls because the amount of growth cannot be secured before the snow falls. Therefore, the optimal planting period for obtaining a certain harvested ball weight (200 g or more) with a relatively low risk of moss abraction is limited to a short period from early to mid-October (Figure 1), which is a barrier to expanding the area of the crop.
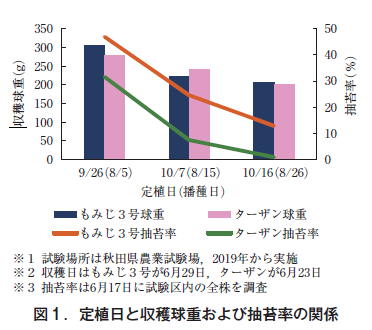
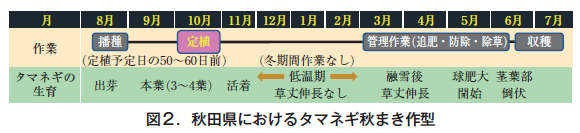
Autumn sowing in this prefecture is planted in October and harvested from June to July, which means that the crop can be grown for about 8 to 9 months (Fig. 2). Because of the long growing season, about three additional fertilizer applications are necessary before and after overwintering, but especially after overwintering, the field becomes muddy due to snowmelt and unseasonable weather, making fertilizer application difficult. In this prefecture, a single application of onion fertilizer requires 0.8 hours of labor per 10 a. In addition, the timing of onion fertilizer application competes with paddy field work, so labor saving is essential (Department of Agriculture, Forestry and Fisheries, Akita Prefecture, 2021).
Fertilizer with regulated fertilizer efficacy that allows full basal application is commonly used for paddy rice, and similar fertilizers have been developed for fall-planted onions. A report from Iwate Prefecture showed that the total basal application of fertilizer to fall-planted onions resulted in yields comparable to conventional fertilizer systems (Iwate Prefectural Agricultural Research Center, 2022). Although our prefecture is located at about the same latitude as Iwate Prefecture, it is necessary to clarify the applicability of this fertilizer because of different climatic conditions such as amount of sunlight and snow cover.
In order to reduce the amount of fertilizer applied, we investigated a labor-saving fertilizer application technique using a fertilizer with regulated fertilizer effect in the autumn sowing of onion in this prefecture, both in the field and in the agricultural experiment station. This study was conducted in FY2021 and FY2022 under the contract with Zen-Noh Fertilizer Co.
Test Methods and Results
(1) On-site testing
(1) Testing method
The test field was located in Oga City, Akita Prefecture, Japan, on the west side of Oogata Village across the western inheritance channel of Hachiro Lagoon. The soil is non-allophenic black-bok soil.
This trial was conducted for two years, planting in 2021 (hereinafter referred to as "trial 1") and 2022 (hereinafter referred to as "trial 2") (Table 1). Seedling containers were 448-hole pots (manufactured by Minoru Sangyo Co., Ltd.), seedling medium was binder medium (manufactured by Minoru Sangyo Co., Ltd.), and seedling management was in accordance with local practices. The planting density was 200 cm between rows, 10.5 cm between plants, and the number of plants was 1,905 plants/a.
The company decided to
For the selection of test fertilizers, a preliminary test was conducted from 2020 to 2021 before the main study. Hara (2020) stated that nitrogen leaching of coated urea is suitable for mathematical modeling in the temperature range of 5 to 40°C and is also suitable for winter cropping, so simulations were conducted using soil temperature data obtained in preliminary trials. The results showed that the sigmoidal LPS30 and LPS40 continued to produce fertilizer until after wintering, and the leaching was almost completed before harvesting.

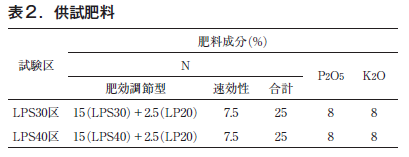
However, LPS30 and LPS40 alone were not sufficient to ensure fall growth, so linear LP Coat 20 and a fertilizer with a fast-acting nitrogen component were tested (Table 2). 25, 8, and 8 kg of N, P2O5, and K2O, respectively, were applied per 10 a. All fertilizer was applied as basal fertilizer. The trial was conducted in triplicate, and four plants per plot were examined for growth data such as grass height and harvested ball weight. The test fertilizers were buried 10 cm below the ground surface in the same field, and data on nitrogen leaching rate and simulation were provided by JCM Agri Co.
(2) Test results
(a) Average temperature and soil temperature
The average temperature (Amedas Point Oga) during the growing season (October to June of the following year), including the period of preliminary trials starting in 2020, was low from November to December in 2020 among the three years, 8.4°C in November and 1.6°C in December before overwintering; in 2021, the temperature was high in October and 14.6°C (Figure Figure 3).
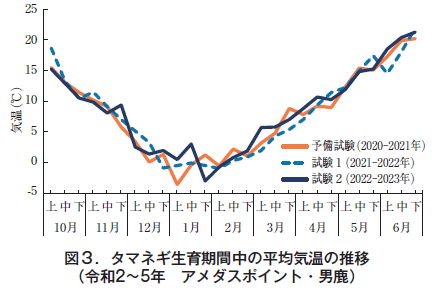
After overwintering, the mean temperature in March was as low as 4.0°C in 2022, but the annual difference was 6.2°C in 2023.
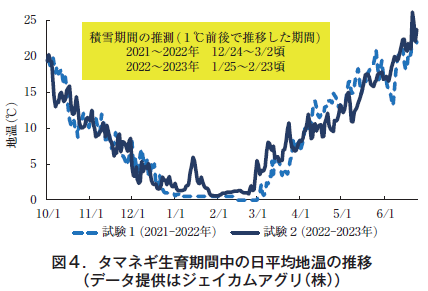
The average daily soil temperature during the onion growing period remained around 1°C from December 24, 2021 to March 2, 2022 in test 1, and from January 25 to February 23, 2023 in test 2 (Figure 4). During this period, the temperature remained almost constant day and night, suggesting that the area was under snow cover.
Although the details of the snow cover are not known because the snow cover was not observed at any of the amedas points (Oga and Oogata) near the test site, the data from Akita City, Akita Prefecture, shows that the consecutive days of 0 cm snow cover were March 9, 2022 and February 27, 2023, which are generally consistent with the estimated dates based on the local ground temperature data. The average monthly ground temperature was 0.5°C. The mean monthly ground temperature in March 2022 was 4.1°C lower than that in 2023 (6.6°C), and in April 2022 was 12.0°C higher than that in 2023 (10.2°C), showing a large difference by year.
(b) Nitrogen leaching of buried fertilizer
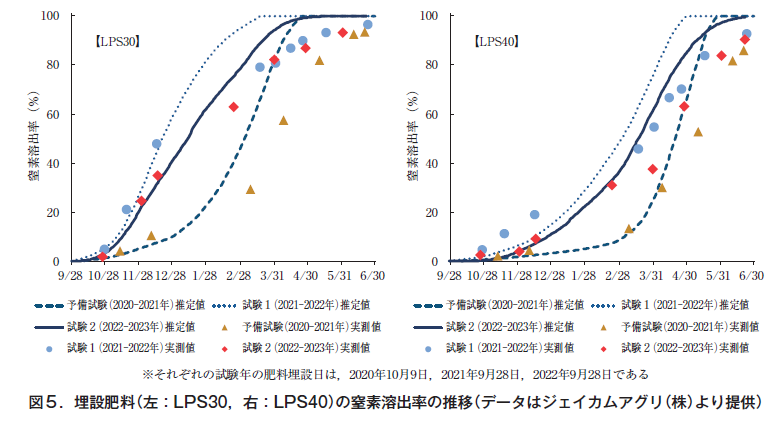
Although fertilizer burial and survey dates varied from year to year, the nitrogen leaching rate of LPS30 was 101 TP3T on December 9, 2020 and 581 TP3T on April 8, 2021 after overwintering in the preliminary test. In Study 1, 481 TP3T was eluted on December 14, 2021, and 811 TP3T on April 1, 2022, about half before overwintering. In test 2, 35% was eluted on December 15, 2022, and 82% on March 31, 2023, which were less eluted before overwintering than in test 1, but were similar after overwintering (Figure 5).
The nitrogen elution rate of LPS40 was 41 TP3T on December 9, 2020 and 301 TP3T on April 8, 2021 after overwintering in the preliminary test. In Study 1, 191 TP3T was eluted on December 14, 2021, and 551 TP3T on April 1, 2022. In Study 2, 91 TP3T was eluted on December 15, 2022, and 381 TP3T on March 31, 2023 (Figure 5).
(c) Effect on grass height
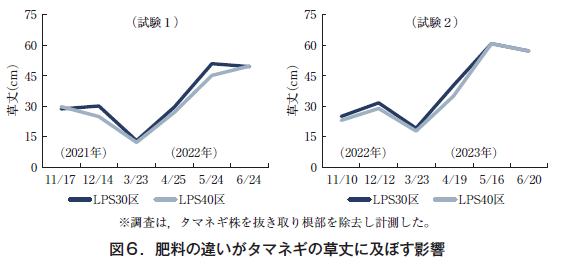
In test 1, grass height in LPS30 was 30 cm on December 14, 2021, which was larger than that in LPS40 (25 cm). After over-wintering, the LPS30 plot remained larger until May 24, 2022, but by the harvest season on June 24, growth had caught up and the LPS30 and LPS40 plots were equal at 49 cm and 50 cm, respectively (Fig. 6).
In test 2, the LPS30 plot remained larger until April 19, 2023 after overwintering, but by harvest time on June 20, the LPS40 plot caught up with the LPS30 plot, and grass height was equal to 57 cm for the LPS30 and LPS40 plots (Fig. 6).
(d) Effect on harvested ball weight and yield
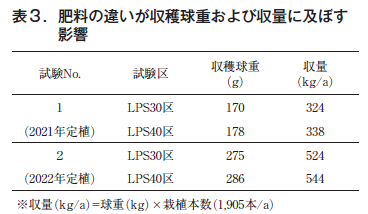
The harvested ball weights were 170 g and 178 g for the LPS30 and LPS40 treatments in Trial 1 and 275 g and 286 g for the LPS30 and LPS40 treatments in Trial 2 conducted the following year, respectively, which were similar in both years. The following year, in Trial 2, the LPS30 and LPS40 treatments were equivalent in weight, with 275 g and 286 g, respectively, in both years.
(2) In-situ testing
(1) Testing method
From 2022 to 2023, trials were conducted at the Akita Prefectural Agricultural Experiment Station (Akita City, Akita Prefecture) to examine the effects of combining fertilizer with supplementary fertilizer. The soil was a non-allophenolic black-bok soil.
The test seed was "Momiji No. 3", the sowing date was August 12, 2022, and the planting date was October 6, 2022.
Seedling containers were 448-hole pots, and seedling medium was Genki-kun green onion medium (manufactured by Katakura Co-op Agri Co., Ltd.). Leaves were pruned three times (September 7, September 22, and September 30), and the seedlings were planted using a hand transplanter. The planting density was 140 cm between rows, 12.5 cm between plants, and the number of plants was 2,286 plants/a. The treatment was applied without replications.
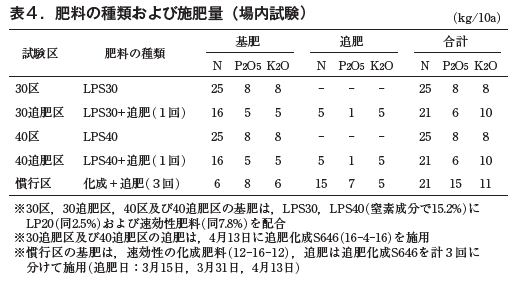
The test plots are shown in Table 4. The fertilizers used were the same as in the field test. The amount of basal fertilizer per 10 a in the plots where LPS30 and LPS40 were applied (plots 30 and 40) was 25 kg of nitrogen, the same amount as in the field test. In the LPS30 + fertilizer and LPS40 + fertilizer plots (hereafter referred to as "30-fertilizer" and "40-fertilizer" plots), the conventional amount of base fertilizer (6 kg of nitrogen) plus two additional applications of fertilizer after overwintering (5 kg/application x 2 = 10 kg) was added, for 16 kg. The conventional plot received three additional fertilizer applications after overwintering. Growth data such as grass height and harvested ball weight were also examined in this trial.
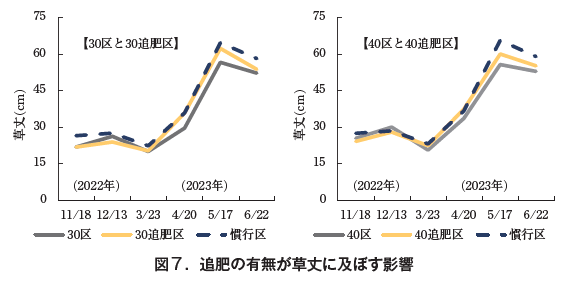
(2) Test results
On December 13, 2022, grass height was greater in the 30 and 40 compartments than in the respective fertilizer compartments, but after April 20, 2023, grass height was greater in the 30 and 40 compartments. Compared to the conventional treatment, the height of the 30-fertilized and 40-fertilized treatments was the same until April 20, but after May 17, the conventional treatment was larger (Fig. 7). The rate of water spot extraction was 2.11 TP3T in the 30-fertilized treatment, which was slightly higher than that in the other treatments. The harvested ball weight was 246 g in the 30-fertilized area, which was larger than that in the 30 area (202 g), indicating the effect of fertilization. 251 g in the 40-fertilized area was equivalent to 247 g in the 40 area. 30 area was smaller than 259 g in the conventional area, but the ball weights in the 30, 40 and 40 areas were almost equivalent to those in the conventional area (Table 5).
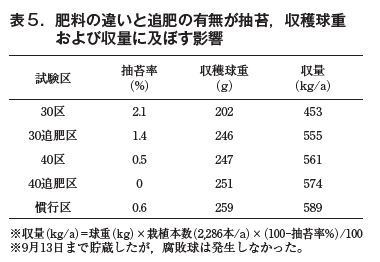
Summary and Discussion
The results of this study showed that the use of controlled-release fertilizer as base fertilizer eliminated the need for additional fertilizer application and resulted in a harvested ball weight of more than 200 g. However, as observed in Tests 1 and 2, even with the same amount of fertilizer applied, there were large annual variations and a difference of more than 100 g in harvested ball weight. However, as observed in Tests 1 and 2, even with the same amount of fertilizer applied, there was a large annual variation and a difference of more than 100 g in harvested ball weight, suggesting that growing only with controlled-release fertilizer is risky and difficult.
The cause is the influence of low temperatures and snow cover from fall to spring. According to a report from Nagasaki Prefecture, nitrogen absorption in onion occurs gradually during winter and increases rapidly when the average daily soil temperature is around 11°C (Hirayama, 2017). Although nitrogen absorption was not measured in this study, data from field trials indicate that soil temperatures generally reach 10°C or higher from April, and nutrient absorption from December to March is considered to be low. However, in this study, the measured nitrogen leaching rate from December to March of the following year was approximately 501 TP3T for LPS30 and 301 TP3T for LPS40. In the case of the spring of 2022, when the snow cover was long and the post-winter temperature was low, the onion growth did not progress even though fertilizer was leached, which delayed the beginning of nutrient absorption and resulted in a smaller harvested ball weight.
The simulation of leaching of buried fertilizers using soil temperature data showed different results from the actual measurements. As mentioned earlier, a temperature range of 5°C or higher is considered to fit the mathematical model, but in this test, the soil temperature was below 5°C from December onward, and furthermore, under snow cover, the soil temperature remained around 0°C. Therefore, it was considered difficult to simulate the use of coated fertilizers during winter in this prefecture.
The field test was conducted in Oga City, Akita Prefecture, an area with relatively little snow cover in the prefecture. However, as the snow cover increases and the snow disappears later inland, the variation in growth and fertilizer leaching is expected to be even greater in other areas of the prefecture.
The results of the field trial showed that the harvested ball weights were higher when two additional fertilizer applications were made to the conventional amount of base fertilizer and a "linking" fertilizer application was made in early April, rather than when the entire amount of base fertilizer was applied. This fertilization method is thought to reduce the need for one additional application of fertilizer after overwintering, thus reducing the need for additional fertilizer.
On the other hand, the use of plastic-coated LP-coated fertilizers is expected to change in the future because of the problems of residues of coated shells on farmland and runoff into the ocean (ZEN-NOH, etc., 2022). Currently, a plastic-free slow-release nitrogen fertilizer, CDU fertilizer, is being tested for post-wintering fertilizer application to reduce the number of fertilizer applications.
Acknowledgements
Ltd. for providing and managing the field sites, and National Agricultural Cooperative Akita Prefecture Headquarters, Akita Namahage Agricultural Cooperative, JCAM Agri Co. We would like to express our deepest gratitude to them.
References
(1)農林水産省.
令和4年産都道府県別・品目別の作付面積,収穫量及び出荷量.(2023)
(2)秋田県農林水産部.
作物別技術・経営指標(2020年版).P174-175.(2021)
(3)岩手県農業研究センター.
秋まきたまねぎ栽培における基肥窒素量減肥の可能性と追肥開始時期.
岩手県農業研究センター試験研究成果書.(2022)
(4)原嘉隆.
被覆尿素肥料からの窒素溶出速度における低温域を含めた温度依存性.
日本土壌肥料学会雑誌.P366-373.(2020)
(5) Japan Meteorological Agency. Meteorological observation data.
https://www.jma.go.jp/jma/index.html
(6)平山裕介.
諫早湾干拓地における中晩生タマネギの生育の推移と日平均気温・地温の関係からみた防除時期と追肥時期.
長崎県農林技術開発センター研究報告.P1-9.(2017)
(7)全国農業協同組合連合会,全国複合肥料工業会,日本肥料アンモニア協会.
緩効性肥料におけるプラスチック被膜殻の海洋流出防止に向けた取組方針.(2022)
Soil Management and Fertilization of Blueberries in Iwate Prefecture, Japan
National Federation of Agricultural Cooperative Associations, Iwate Headquarters
Horticulture Department, Horticulture Specialty Products Division
佐々木 仁
Introduction
Blueberry cultivation in Iwate Prefecture began in 1979, when the late Professor Emeritus Kiyoshi Yokota of the Faculty of Agriculture, Iwate University, energetically promoted the cultivation of blueberries, and at its peak in 2017, the total cultivated area reached 56.7 ha (Agricultural and Forestry Statistics). However, due to the aging of producers and the lack of new cultivators, the area has gradually decreased since then.
The decrease in cultivated area is not only due to the aging of growers, but also to the difficulty of cultivation, as blueberries do not grow well even after planting due to their narrow soil adaptability and susceptibility to wet and dry conditions, and do not reach maturity. This paper introduces the current status of soil management and fertilization of blueberries in Iwate Prefecture and future measures.
2. blueberry planting and soil management
Blueberry varieties can be broadly divided into three strains: northern highbush blueberry, southern highbush blueberry, and rabbiteye blueberry. However, the highbush blueberry is not a good candidate for cold climate cultivation. However, because highbush blueberries have mainly fine roots and narrow soil adaptability, sufficient soil improvement and subsequent soil management are important when planting them.
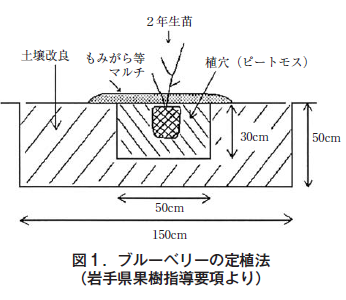
Blueberry is a fruit tree belonging to the family Rhododendronaceae, and the soil suitable for cultivation is rich in organic matter and acidic with a pH range of 4.0 to 5.5 (optimum is 4.3 to 4.5). Therefore, the pH should be checked before planting, and if it is too high, the pH should be corrected using sulfuric acid. In addition, acidic organic materials (peat moss, etc.) should be added to the planting hole to promote seedling establishment (Figure 1), and after planting, the area around the plants should be mulched with woody bark, chips, or fir (Figure 2) to prevent soil drying and weed infestation.

After planting, it is advisable to periodically mulch with woody material to replenish organic matter (Figure 3). In fruit tree areas, apple branches can be used as organic matter, both for soil improvement at planting and for under-canopy mulch after planting. However, since apple branches contain relatively high calcium content, the pH of the soil tends to become high after prolonged use as mulch, so the soil pH should be checked periodically. Most organic materials such as hardwood and softwood chips can be used as they are, but organic materials such as unripe manure or fresh mushroom bed waste can be harmful and should be avoided.

Generally, the most common failure of blueberry cultivation is planting in former rice paddy fields, where even if the soil is acidic, the roots cannot spread sufficiently because of its clay content. Furthermore, if drainage measures are inadequate, the root zone may become waterlogged for a long period of time, resulting in the weakening and death of the tree at the young tree stage. For this reason, it is desirable to plant in culverts or culverts in former rice paddies (Figure 4), and if necessary, to plant in higher rows. On the other hand, on the other hand, on black-brick soils with soft and humus-rich soil, even simple soil amendments can be used for vigorous growth, and there have been many cases where cultivation has been successful.

Types of fertilizers used for blueberries and their application methods
Blueberry prefers chisso as a nutrient, and ammonium sulfate is often applied as a basic fertilizer because of its excellent ammonium-form chisso fertilizer effect. In addition, since ammonium sulfate is high in sulfur, it is also highly effective in lowering soil pH and maintaining acidity, making it suitable for blueberry cultivation. On the other hand, if the soil is too low below the optimum pH (pH 5 or lower), it is better to use urea, which is almost pH neutral and easily supplies ammonium nitrate. Although these fertilizers are single-chloroethylene fertilizers, compound fertilizers containing phosphoric acid, potassium, and trace elements in addition to chloroethylene are manufactured and sold (Table 1).
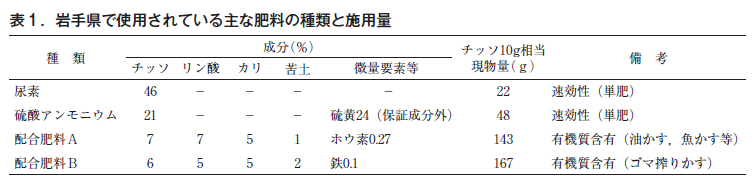
Blueberry is sensitive to nitrogen, and either too much or too little can cause a variety of symptoms on leaves, branches, and shoots, resulting in decline and death. However, in any case, avoid applying too much fertilizer at once, and adjust fertilizer according to the growth of shoots and leaf color, especially for young trees.
Dr. Yokota, mentioned above, has developed a thiofertilization standard for blueberries based on a USDA text (Table 2), and Iwate Prefecture follows the standard. In addition to base fertilizer in the spring, additional fertilizer is applied in June, when shoots are actively growing, and in July, just before fruit maturity, and no fertilizer is applied thereafter. This is because fertilization with nitrogen fertilizer after August is very harmful, as it reduces the freezing resistance of the tree, causing branches to die due to low temperatures and cold winds the following spring (Fig. 5) and inhibiting the development of new shoots (Fig. 6). This disorder is especially likely to occur when the tree is too vigorous, so it is important to adjust the amount of fertilizer applied according to growth (there are also differences among varieties).
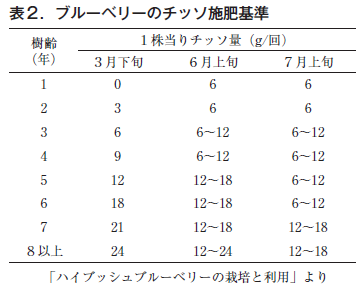

The actual amount of nitrogen fertilizer applied per tree exceeds 10 g when the tree is in its third year (Table 2), but the actual amount varies greatly depending on the nitrogen content of the product (Table 1), so care should be taken when applying fertilizers. As the trees get older, a considerable amount of fertilizer is applied at a time, so the amount should be adjusted according to the growth conditions of the trees, and in some cases, the number of applications should be increased and the amount applied per application should be reduced.
4. for the future
Because of the limited availability of suitable soils for blueberry cultivation, there are many cases of farmers abandoning cultivation due to poor growth or struggling with unsuitable orchard sites.
One solution to this problem is to use large amounts of wood chips or bark instead of soil, a method that has been adopted in some areas of Iwate Prefecture. In Iwate Prefecture, this method is also being used in some cases, and it shows that despite the use of raw chips, rooting is vigorous (Fig. 7) and above-ground growth is good. The problem is that the chips gradually decompose and turn to leaf litter, which requires periodic replenishment, although the degree of replenishment depends on the species of hardwoods and softwoods, and the particle size of the chips. In addition, since the occurrence of soil fertility and fertilizer efficacy may differ from those of ordinary soils, it is necessary to examine whether the current fertilizer standards can be maintained (fertilizer amount, timing of application, etc.).

Another possible solution is the use of rootstock. By grafting highbush blueberries onto rabbit-eye blueberries, which have robust roots and vigorous growth (Figure 8), it has become possible to alleviate the disadvantage of limited soil adaptability (Figure 9), and recently grafted seedlings have been sold. Although there are some problems, such as the unit price of seedlings and the occurrence of the brown fly from the rootstock, grafting is considered to be an effective means of ensuring the healthy cultivation of highbush blueberries under the circumstances of global warming.
The trees grown on rootstock have strong roots and absorb more fertilizer than those grown on woodchips, which may cause them to grow taller, requiring caution in the amount of nitrogen applied. Therefore, it is possible that the current fertilizer standards for highbush blueberries, as in the case of woodchips, do not apply the same amount of fertilizer to highbush blueberries.
In the future, when new cultivation methods are introduced, it will be impractical to develop fertilizer application standards for each new method. Therefore, we would like to see the development of a method to determine the amount of fertilizer to be applied based on the current fertilizer application method, using the diagnosis of the tree phase based on the elongation of shoots and leaf area.


References
●岩手ブルーベリーの会
ハイブッシュブルーベリーの栽培と利用,令和3年
●令和3年度岩手県果樹指導要項
●平成27年度岩手県農業研究センター試験研究成果
Use of Organic Materials in Blueberry Cultivation
No Soil - No. 39
慣行農業の養分源・化学肥料の課題
-原料の資源枯渇や生産のエネルギー問題-
前 ジェイカムアグリ株式会社
北海道支店 技術顧問
松中 照夫
In the previous issue, we discussed the weakness of nutrient-recycling organic agriculture, which requires the preparation of farmland for the production of compost as a self-sufficient nutrient source. In this issue, we will discuss chemical fertilizers used as a source of nutrients in conventional agriculture.
Chemical fertilizers are produced from mined ores. As long as this process is repeated, resource depletion is certain to occur, just as it does with fossil fuels. This is because the mineral resources used as raw materials for fertilizers are non-renewable. In the following, I would like to outline the problem of resource depletion in the order of phosphorus, potassium, and nitrogen, and point out that even in conventional agriculture, there is a major weakness in the source of nutrients.
1. phosphorus - oligopoly of producing countries causes concerns about stable supply
Chemical fertilizers began with the production of superphosphate lime by treating animal bone meal with sulfuric acid. However, as demand for fertilizers increased, animal bones became scarce, and in Europe, not only animal bones but also the bones of soldiers who had fallen on the battlefield were used. It was not until the discovery of phosphate ore in Florida and Carolina in the late 19th century that the raw material for phosphorus fertilizer was replaced by ore.
Current phosphate ore is highly unevenly distributed around the globe: global phosphate ore reserves in 2023 were 74 billion tons (U.S. Geological Survey, 2024), 68% of which are located in Morocco. Annual production of phosphate ore is 220 million tons. China is the world's largest producer, accounting for 41% of the world's output. China, Morocco, and the United States together account for 66% of total production.
After Russia's invasion of Ukraine, countries around the world have increased the acreage of food production and the amount of fertilizer applied due to a sense of crisis over food security. As a result, the demand for fertilizers in general has increased. It is estimated that the supply of phosphorus will not be able to meet this demand until around 2040, after which demand is expected to exceed supply (Cordell et al., 2013). Based on current estimates, phosphorus ore resources are expected to be depleted in 30 years at the earliest, and in 300 years at the longest. Furthermore, since mining of high-grade phosphate ore has advanced considerably, low-grade phosphate ore will have to be used in the future. The cost of extracting phosphorus from low-grade phosphorus ores after mining is high, and higher prices are inevitable.
Low-grade phosphate ores often contain heavy metals such as cadmium and arsenic, and radioactive materials such as radium and thorium as impurities. Therefore, there is concern that mining may pollute the surrounding environment.

2. potassium - Discovery of an ore that could save forests from extinction
The source of potassium was ash from burning forests. The ash was mixed with water and the potassium was extracted from the supernatant liquid (this is called potassium potassi). Potassium-containing potash ore was discovered deep in the salt deposits of central Germany in 1860.
Potassium was used more as a raw material for gunpowder than for fertilizer. In the early 19th century, during the Napoleonic Wars, France actively used potash for gunpowder production. If potash had not been discovered in the mid-19th century, the forests of Europe would have been destroyed as forests were cut down to produce the potash needed to manufacture gunpowder. The discovery of potash ore was indeed a great discovery that saved Europe's forests from extinction. It was not until 1840, when the importance of potassium as a fertilizer ingredient was recognized, that potash ore began to attract attention as a raw material for fertilizer.
Germany currently produces 7% of the world's 39 million tons of potash ore in 2023, ranking it fifth in the world (U.S. Geological Survey, 2024). Currently, Canada, Russia, China, and Belarus produce 33%, 17%, 15%, and 10% of the world's potash ore, respectively, and including Germany's 7%, these five countries produce 82% of the world's potash ore. Like phosphate, potash ore is unevenly distributed around the globe.
According to the U.S. Geological Survey (2024), potassium reserves in 2023 are at least 11 billion tons of recoverable ore. In terms of potassium (K2O equivalent) reserves, the same five countries as the producing countries account for 79% of the world's potassium reserves. If this recoverable ore volume is mined at the current ore production rate, the service life is approximately 280 years. However, as in the case of phosphate ore, a decline in production efficiency is inevitable due to the gradual deterioration of mining economics and the potassium content of the ore. As in the case of phosphate ore, the depletion of the resource will come earlier than expected.
Nitrogen - Fertilizer raw materials are shifting from mineral resources to airborne nitrogen.
In the 1840s, guano containing nitrogen and phosphorus was exported from Peru to Europe. In 1898, Crookes (1832-1919), who became president of the British Academical Society, stated in his inaugural address that "the deposits of Chilean nitre will be exhausted in the near future. It is, therefore, a serious and urgent matter to convert the unlimited amount of nitrogen in the air into a fertilizer that can be used by plants.
Inspired by this, research on the industrial use of nitrogen gas from the air as a raw material for fertilizers was promoted. This was made possible by the German ammonia synthesis method, the Haber-Bosch process. Haber (1868-1934) was awarded the Nobel Prize in Chemistry in 1918 for this work, and Bosch (1874-1940) was awarded the Nobel Prize in Chemistry in 1931 for his high-pressure chemistry. Smil (1943- ) said, "The greatest invention of the 20th century is not the airplane, nuclear power, space flight, television, or computers, but the industrialization of ammonia synthesis. Without it, the population would not have increased from 1.6 billion to 6 billion in the 20th century" (Smil, 2001).
Since nitrogen is derived from nitrogen gas in the air, it is often thought that there is no concern about depletion of the raw material. However, this ammonia synthesis method also has its problems. The hydrogen gas used to react with nitrogen gas is produced by high-temperature decomposition of hydrocarbons contained in heavy oil, crude oil, coke gas, natural gas, naphtha, etc. This process requires an enormous amount of energy. It has been pointed out that this energy consumption amounts to several percent or more of the energy consumed by all mankind (Ashida et al., 2022). Another problem is that the recovery rate of ammonia synthesis is as low as 30%, despite the consumption of such a large amount of energy. As long as the Haber-Bosch process uses fossil fuels, a finite resource, as its energy source, ammonia synthesis cannot be continued sustainably.
Recently, results overcoming this problem have been published. Ashida et al. succeeded for the first time in the world in synthesizing ammonia from nitrogen gas in air using visible light energy under mild reaction conditions at room temperature and pressure (Ashida et al., 2022). This reaction is catalyzed by an iridium photo-redox catalyst and a molybdenum catalyst. We look forward to the industrialization of this synthetic reaction. If this is realized and if catalyst resources are sufficient, the problem of nitrogen fertilizer resource depletion may be overcome.
As we have seen, all nutrients provided to farmland are finite resources. Finding ways to reuse these nutrients without wasting them is an extremely important task for us as we face resource depletion.